Answer: 169.3 g of
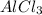 will be produced
will be produced
Step-by-step explanation:
To calculate the moles, we use the equation:
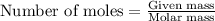
moles of
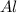
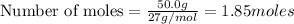
moles of
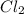
According to ideal gas equation:

P = pressure of gas = 1 atm (STP)
V = Volume of gas = 42.7 L
n = number of moles = ?
R = gas constant =
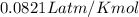
T =temperature =
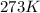 (at STP)
(at STP)
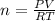
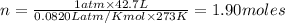
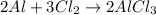
According to stoichiometry:
3 moles of
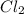 reacts with = 2 moles of aluminium
reacts with = 2 moles of aluminium
Thus 1.90 moles of
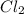 reacts with=
reacts with=
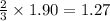 moles of aluminium
moles of aluminium
Thus
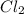 is the limiting reagent as it limits the formation of product.
is the limiting reagent as it limits the formation of product.
As 3 moles of
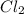 give = 2 moles of
give = 2 moles of
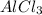
Thus 1.90 moles of
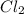 give =
give =
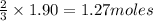 of
of
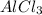
Mass of
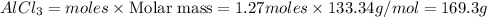
Thus 169.3 g of
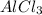 will be produced from the given masses of both reactants.
will be produced from the given masses of both reactants.