Answer:
Q = 976.5 J.
Step-by-step explanation:
Hello there!
In this case, according to the thermodynamic definition of the heat in terms mass, specific heat and temperature change, we can write:
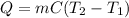
Thus, by plugging in the given data, we obtain:
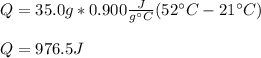
Regards!