Answer:
1.16 as a ratio and 1.1 as difference.
Step-by-step explanation:
Hello there!
In this case, since the pH stands for the relative strength of the hydrogen ions in a solution, it is widely known that the pH of the ocean is about 8.1 due to the dissolved HCO3^- ions dissolved in it. On the other hand the pH of pure water is known as 7.0 as the only neutral solution.
Thus, by dividing the pH of the ocean by the pH of the pure water, we can calculate the units or ratio between them:
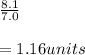
Or also the difference separating them:
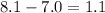
Best regards!