Answer:
Six atoms of calcium, four of phosphorous and sixteen of oxygen for a total of twenty six
Step-by-step explanation:
Hello there!
In this case, according to the molecular formula of the two moles of calcium phosphate:
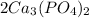
Thus, in order to calculate the atoms of each atom, it is necessary to multiply the two in front of the formula by the subscripts in the reaction:
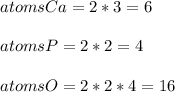
Thus, we obtain six atoms of calcium, four of phosphorous and sixteen of oxygen for a total of twenty six.
Best regards!