Answer:
a) 2250 J
b) 0 J
c) 2250 J
Step-by-step explanation:
a) Since, the process is isochoric
the change in internal energy
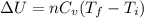
Here, n = 0.2 moles
Cv = 12.5 J/mole.K
We have to find T_f so we can use gas equation as
![(P_1V_1)/(P_2V_2) =(T_i)/(T_f)\\Since, V_1=V_2 [isochoric/process]\\\Rightarrow (P_(atm))/(4P_(atm)) = (300)/(T_f) \\\Rightarrow T_f = 1200 K](https://img.qammunity.org/2022/formulas/physics/college/r7t8slwj3qw8z9agoyuws5xub50zu6tp6m.png)
So,
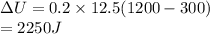
b) Since, the process is isochoric no work shall be done.
c) By first law of thermodynamics we have
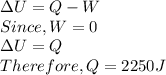
Since, Q is positive 2250 J of heat will flow into the system.