Answer:
Average Density = 1.07 g/cm³
Step-by-step explanation:
The average density of the whole mixture is given by the following formula:
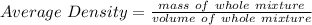
where,
volume of whole mixture = volume of water + volume of acid
volume of whole mixture = 100 cm³ + 50 cm³ = 150cm³
mass of whole mixture = mass of water + mass of acid
mass of whole mixture = (volume of water)(density of water) + (volume of acid)(density of acid)
mass of whole mixture = (100 cm³)(1 g/cm³) + (50 cm³)(1.2 g/cm³)
mass of whole mixture = 160 g
Therefore,
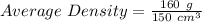
Average Density = 1.07 g/cm³