Answer:
V = 11.2 L
Step-by-step explanation:
Hello there!
In this case, according to the ideal gas equation:
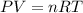
It is possible to compute the volume as shown below:
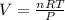
Whereas the moles are computed are computed given the mass and molar mass of oxygen:
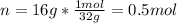
Now, since the STP stands for a temperature of 273.15 K and a pressure of 1 atm, the resulting volume is:

Best regards!