Answer:
4118.2
Step-by-step explanation:
Given: 29 moles of na²
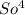
To find: In grams.
Solution: Wrong
If 1 na²
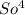 is 142 grams, then 29 grams will be found if a set of fraction has been set up;
is 142 grams, then 29 grams will be found if a set of fraction has been set up;
Let
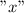 be the variable
be the variable
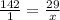
Cross multiply
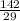
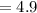
Correct Solution
Multiply 29 by 142 = 4118