Answer:
D. neon.
Step-by-step explanation:
Hello there!
In this case, according to the following bonding:
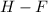
It is possible to bear to mind the fact that hydrogen has one valence electron whereas fluorine has seven. Moreover, once they bond, we understand fluorine takes the only hydrogen's valence electron to attain the electron configuration of the former's contiguous noble gas which is D. neon.
Best regards!