Answer:
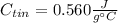
Step-by-step explanation:
Hello there!
In this case, for this calorimetry problem, we can notice that the heat evolved by the hot tin is gained by the cold water as the calorimeter is perfectly isolated, so we can write:
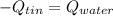
Thus, by defining the heats in terms of mass, specific heat and temperatures, we get:
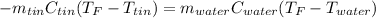
Now, since we are asked for the specific heat of tin, we solve for it as shown below:
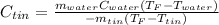
Thus, when we plug in, we obtain:
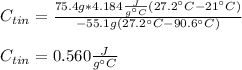
Regards!