Answer:
0.0300 moles of H₂
Step-by-step explanation:
The original equation is PV = nRT. We need to change this to show moles (n).
n =
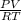
It's important to convert your values to match the constant (r) in terms of units.
30.0 kPa = 0.296 atm
2500 mL = 2.50 L
27 °C = 300 K
Now, plug those values in to solve:
n =
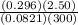 - for the sake of keeping the problem clean, I didn't include the units but you should just to make sure everything cancels out :)
- for the sake of keeping the problem clean, I didn't include the units but you should just to make sure everything cancels out :)
Finally, you are left with n = 0.0300 moles of H₂