Answer:
44.2 L
Step-by-step explanation:
Use Charles Law:
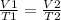
We have all the values except for V₂; this is what we're solving for. Input the values:
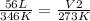 - make sure that your temperature is in Kelvin
- make sure that your temperature is in Kelvin
From here, we need to get V₂ by itself. To do this, multiply by 273 on both sides:
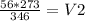
Therefore, V₂ = 44.2 L
It's also helpful to know that temperature and volume are linearly related. So, when temperature drops, so will volume and vice versa.