Answer:
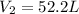
Step-by-step explanation:
Hello there!
In this case, by bearing to to mind the given conditions, it is firstly possible to determine the initial volume of the closed system via the ideal gas equation:
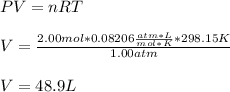
Which is V1 in the Charles' law:
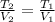
And of course, T1 is 298.15 (25+273.15). Therefore, by solving for V2 as the final volume, we obtain:
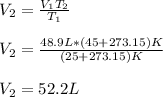
Best regards!