Answer:
HClO3>HClO2>HClO>HBrO
Step-by-step explanation:
Hello there!
In this case, in agreement to the given information and the reported acidic dissociation constant (Ka) for these acids, which are shown below:
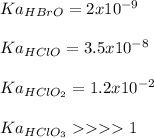
In such a way, since the Ka of chloric acid, HClO3 is greater than 1, we infer it is a strong acid so it is the strongest, next we have HClO2, then HClO and the weakest would be HBrO as its Ka is the smallest.
Thus, the order would be:
HClO3>HClO2>HClO>HBrO
Best regards!