Answer:
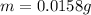
Step-by-step explanation:
Hello there!
In this case, it is possible to comprehend these mass-particles problems by means of the concept of mole, molar mass and the Avogadro's number because one mole of any substance has 6.022x10²³ particles and have a mass equal to the molar mass.
In such a way, for C₆H₁₂O₆, whose molar mass is about 180.16 g/mol, the referred mass would be:
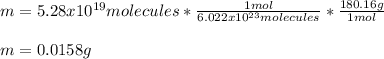
Best regards!