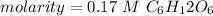Answer:

Step-by-step explanation:
Molarity is found by dividing the moles of solute by liters of solution.
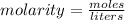
We are given grams of a compound and milliliters of solution, so we must make 2 conversions.
1. Gram to Moles
We must use the molar mass. First, use the Periodic Table to find the molar masses of the individual elements.
- C: 12.011 g/mol
- H: 1.008 g/mol
- O: 15.999 g/mol
Next, look at the formula and note the subscripts. This tells us the number of atoms in 1 molecule. We multiply the molar mass of each element by its subscript.
6(12.011)+12(1.008)+6(15.999)=180.156 g/mol
Use this number as a ratio.
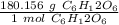
Multiply by the given number of grams.
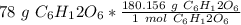
Flip the fraction and divide.
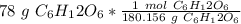
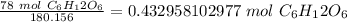
2. Milliliters to Liters
There are 1000 milliliters in 1 liter.
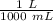
Multiply by 2500 mL.
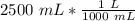
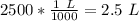
3. Calculate Molarity
Finally, divide the moles by the liters.
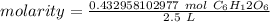
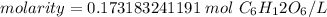
The original measurement has 2 significant figures, so our answer must have the same. That is the hundredth place and the 3 tells us to leave the 7.
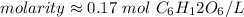
1 mole per liter is also equal to 1 M.