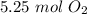Answer:
5.25 moles
General Formulas and Concepts:
Chemistry
Atomic Structure
Aqueous Solutions
Stoichiometry
- Analyzing reactions RxN
- Using Dimensional Analysis
Step-by-step explanation:
Step 1: Define
[RxN - Balanced] 2KClO₃ (s) → 2KCl (s) + 3O₂ (g)
[Given] 3.50 mol KClO₃
[Solve] mol O₂
Step 2: Identify Conversions
[RxN] 2 mol KClO₃ (s) → 3 mol O₂ (g)
Step 3: Stoichiometry
- [DA] Set up conversion:
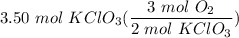
- [DA} Multiply [Cancel out units]: