Answer:
15.2 grams of calcium chloride are produced and HCl is the limiting reactant.
Step-by-step explanation:
Hello there!
In this case, according to the described scenario, it is possible to realize that the reaction between hydrochloric acid and calcium hydroxide is:
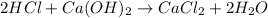
Whereas there is a 2:1 mole ratio of the acid to the base. In such a way, with the given masses, we can compute how much calcium chloride product is produced due to the chemical reaction via stoichiometry:
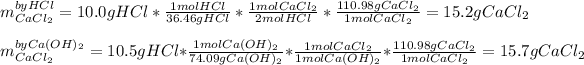
Whereas we infer that the correct amount is 15.2 g since HCl is the limiting reactant as it produces the fewest grams of the desired product. Consequently, the calcium hydroxide is the excess reactant here.
Regards!