Answer:
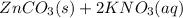
Step-by-step explanation:
Hello there!
In this case, for the referred reactants side, we can evidence that this is a double displacement reaction whereby Zn gets bonded with CO3 and therefore K gets bonded with NO3 to yield:
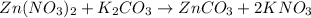
Whereas the KNO3 product is aqueous and the ZnCO3 is solid due to the corresponding solubility rules. Thereby, the answer is the third one.
Regards!