Answer:
Step-by-step explanation:
The rate of weight change can be determined by using the formula:
weight change = weight at the final time - weight at the initial time.
Molarity Weight change
0.0M sucrose → (4.68 - 4.36) = 0.32 g/min
0.4 M sucrose → (4.28 - 4.59) = - 0.31 g/min
0.6 M sucrose → (4.0 - 4.7) = - 0.7 g/min
0.8 M sucrose → (3.7 - 4.5) = - 0.8 g/min
1.0 M sucrose → (4.08 - 5.0) = - 0.92 g/min
Percent weight change =
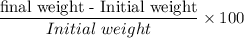
Now;
Molarity Weight change
0.0M sucrose →
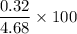 = 6.8 %
= 6.8 %
0.4 M sucrose →
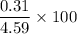 = 8.4 %
= 8.4 %
0.6 M sucrose →
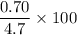 = 14.8 %
= 14.8 %
0.8 M sucrose →
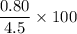 = 17 %
= 17 %
1.0 M sucrose →
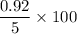 = 18.4%
= 18.4%