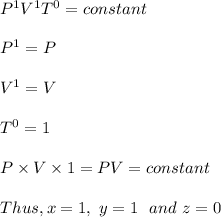Answer:
x = 1, y = 1 and z = 0
Step-by-step explanation:
Given equation;
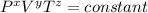
Boyle's law states that at constant temperature, the volume of a fixed mass of gas is inversely proportional to its pressure.
Mathematically the law is written as;
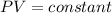
From the given equation, the values of x, y and z that will match this law is calculated as follows;