Answer: The moles needed are 0.01.
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute dissolved per liter of the solution.
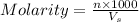
where,
n = moles of solute
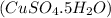 = ?
= ?
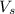 = volume of solution in ml
= volume of solution in ml
Now put all the given values in the formula of molarity, we get
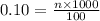
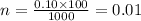
Therefore, the moles needed are 0.01.