Answer:
87.15%
Step-by-step explanation:
To find percent yield, we can use this simple equation
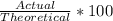
Where "Actual" is the amount in grams actually collected from the reaction, and "Theoretical" is, well, the theoretical amount that should have been produced.
They give us these values, so to find the percent yield, just plug the numbers in.
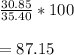
So, the percent yield is 87.15%
An easy trick to remember how to do this is just to divide the smaller number by the bigger number and move the decimal back two places. If you have a percent yield greater than 100%, something is wrong in the reaction.