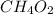Answer:
Step-by-step explanation:
The empirical formula is the simplest whole-number ratio of the elements in a compound to one another. To find the empirical formula given a molecular formula, divide all the subscripts by their greatest common factor.
3, 12, and 6 can all be divided by 3, which makes the empirical formula