Answer:
The new volume is approximately 17.637 liters
Step-by-step explanation:
The given parameters in the question are;
The initial volume of the gas, V₁ = 13.5 Liter
The initial temperature of the gas, T₁ = 248 K
The new temperature of the gas, T₂ = 324 K
A balloon containing gas that experiences an increase in the temperature of the gas content under constant pressure obeys Charles law which states that the volume of a given mass of gas is directly proportional to its Kelvin temperature is expressed mathematically as follows;
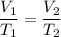
Where;
V₁ = The initial volume of the gas
T₁ = The initial temperature of the gas
V₂ = The new volume of the gas
T₂ = The new temperature of the gas
From the question, we have;
V₁ = 13.5 Liter
T₁ = 248 K
T₂ = 324 K
Therefore, we have;
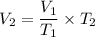
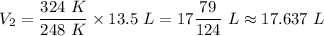
The new volume, V₂ ≈ 17.637 L.