Answer: The total pressure is 7 atm
Step-by-step explanation:
According to Dalton's law, the total pressure is the sum of individual pressures.
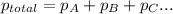
Given :
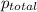 =total pressure of gases = ?
=total pressure of gases = ?
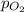 = partial pressure of oxygen = 2 atm
= partial pressure of oxygen = 2 atm
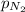 = partial pressure of nitrogen = 4 atm
= partial pressure of nitrogen = 4 atm
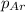 = partial pressure of argon = 1 atm
= partial pressure of argon = 1 atm
putting in the values we get:
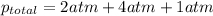
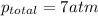
Thus the total pressure is 7 atm