Answer:
A) don't see an x B) x=23 C) x=2; D) x=-1
Explanation:
B) Cube both sides...
![(\sqrt[3]{4+x})^3=3^3](https://img.qammunity.org/2023/formulas/mathematics/high-school/siwo3kr1fqfwlyis9f7rfoitgk46sbbsc0.png) ---> 4+x=27
---> 4+x=27
C) add
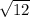 to both sides...
to both sides...
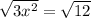 ... square both sides...
... square both sides...
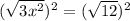 --> 3x^2=12... divide both sides by 3--> x^2=4---> x=2
--> 3x^2=12... divide both sides by 3--> x^2=4---> x=2
D=
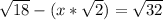 ... divide each side
... divide each side
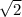 --->
--->
![(√(18)/√(2))- [(x*√(2))/√(2)]= √(32)/√(2)](https://img.qammunity.org/2023/formulas/mathematics/high-school/gdhj917mca2rgcuoiqmmn94drj0hbuqkof.png) --->
--->
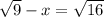 ... and because 9 and 16 are perfect squares our equation now reads---> 3-x=4---> -x=1---> x=-1
... and because 9 and 16 are perfect squares our equation now reads---> 3-x=4---> -x=1---> x=-1