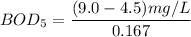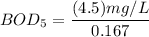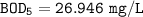Answer:
27 mg/L
Step-by-step explanation:
From the given information:
To determine the 5-day BOD using the formula:
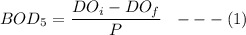
where;
BOD = biochemical oxygen demand
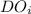 = initial dissolved oxygen
= initial dissolved oxygen
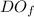 = final dissolved oxygen
= final dissolved oxygen
P = dilution factor
The dilution factor
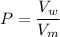
where;
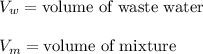
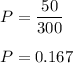
replacing the value of P into equation (1);