Answer:
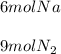
Step-by-step explanation:
Hello there!
In this case, given the chemical reaction:
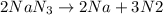
It is possible to evidence the 2:2 mole ratio of the reactant to Na and the 2:3 mole ratio of the reactant to the N2; therefore, the following setups allows us to compute the moles of products:
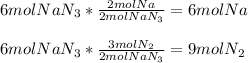
Best regards!