The given question is incomplete. The complete question is:
The combustion of propane (C3H8) in the presence of excess oxygen yields
 and
and
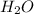
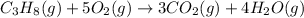
When only 2.5 mol of
 are consumed in order to complete the reaction, ________ mol of
are consumed in order to complete the reaction, ________ mol of
 are produced.
are produced.
Answer: Thus when 2.5 mol of
 are consumed in their reaction, 1.5 mol of
are consumed in their reaction, 1.5 mol of
 are produced
are produced
Step-by-step explanation:
The balanced chemical equation is:
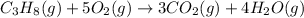
According to stoichiometry :
5 moles of
 produce = 3 moles of
produce = 3 moles of

Thus 2.5 moles of
 will produce =
will produce =
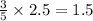 moles of
moles of

Thus when 2.5 mol of
 are consumed in their reaction, 1.5 mol of
are consumed in their reaction, 1.5 mol of
 are produced
are produced