Answer:
4. .02
Step-by-step explanation:
Hello there!
In this case, since the molarity of a solution is defined as the moles of the solute divided by the volume of the solution, we can see we are given the molarity and volume and are asked to compute moles; thus, we can solve as shown below:
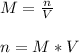
Whereas the volume must be in liters (1 L in this case); in such a way we can plug in the volume and molarity to obtain:
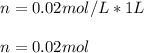
Therefore, the answer is 4. .02 .
Best regards!