Answer:
e. BaSO4 and KCI.
Step-by-step explanation:
Hello there!
In this case, given the reactants side:
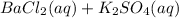
It is possible to realize that after the reaction takes place barium replaces potassium and therefore barium sulfate and potassium chloride are formed:
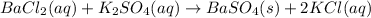
Therefore, the products turn out to be e. BaSO4 and KCI.
Best regards!