Answer:

Step-by-step explanation:
The mass number is the sum of protons and neutrons.
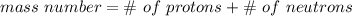
This mystery element's isotope has 8 protons and 10 neutrons. Protons and neutrons are both equal to 1 amu, so 8 protons is 8 amu and 10 neutrons is 10 amu.
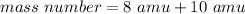
Add.
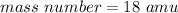
The mass number of this isotope is 18 atomic mass units.