Answer:
0.132 pm
Step-by-step explanation:
De Broglie Wavelength is
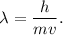
or
wavelength = h / (m * v)
To calculate the De Broglie wavelength of a neutron traveling at 1% the speed of light, you will need to know the mass of the neutron and the speed of light. The mass of a neutron is about 1.675 x 10^-27 kilograms, and the speed of light is about 3.00*10^8 meters per second.
Plugging in these values, you get:
wavelength = 6.626 x 10^-34 J * s / (1.675 x 10^-27 kg * (0.01 * 3.00x10^8 m/s))
Simplifying this equation, you get:
wavelength = 1.3186x10^-13 meters
(1.3186x10^-13 meters) * (1 picometer / 10^-12 meter) =
0.132 picometers