0.455 L (or 455 mL) of 1.60 x
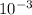 M HCl is needed to neutralize 14.5 mL of a saturated
M HCl is needed to neutralize 14.5 mL of a saturated
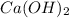 solution.
solution.
To determine the volume of 1.60 x
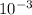 M HCl that is needed to neutralize 14.5 mL of a saturated
M HCl that is needed to neutralize 14.5 mL of a saturated
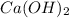 solution, we can first calculate the number of moles of
solution, we can first calculate the number of moles of
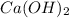 that are present in the solution.
that are present in the solution.
We can do this by using the solubility of
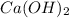 in water (0.185 g/100.0 mL) and the volume of the solution (14.5 mL):
in water (0.185 g/100.0 mL) and the volume of the solution (14.5 mL):
0.185 g
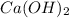 / 100.0 mL * 14.5 mL = 0.026975 g
/ 100.0 mL * 14.5 mL = 0.026975 g
Next, we can use the molar mass of
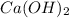 (74.1 g/mol) to convert the mass of
(74.1 g/mol) to convert the mass of
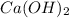 to moles:
to moles:
0.026975 g
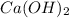 / 74.1 g/mol = 0.000364 mol
/ 74.1 g/mol = 0.000364 mol
Since the reaction between HCl and
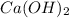 is a 2:1 mole ratio, we can then determine the number of moles of HCl that are needed to neutralize the
is a 2:1 mole ratio, we can then determine the number of moles of HCl that are needed to neutralize the
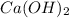 :
:
0.000364 mol Ca(OH)2 * 2 = 0.000728 mol HCl
Finally, we can use the concentration of the HCl solution (1.60 x
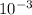 M) and the number of moles of HCl that are needed to determine the volume of HCl that is required:
M) and the number of moles of HCl that are needed to determine the volume of HCl that is required:
0.000728 mol HCl / (1.60 x
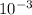 M) = 0.455 L HCl
M) = 0.455 L HCl
Therefore, 0.455 L (or 455 mL) of 1.60 x
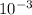 M HCl is needed to neutralize 14.5 mL of a saturated
M HCl is needed to neutralize 14.5 mL of a saturated
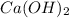 solution.
solution.