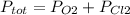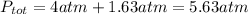Answer:5.63 atm
Explanation: Dalton's Law states that total pressure of a mixture of gasses is equal to the sum of partial pressure of each gas.
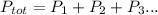
We are given pressure O2 and pressure Cl2.
Step 1: First, convert 3040mmHg to atm by dividing by 760.
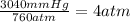
Step 2: Plug your numbers into the equation