Answer: 56.1 g
Step-by-step explanation:
We begin by finding the formula mass of KCl.
- The atomic mass of K is 39 g/mol.
- The atomic mass of Cl is 35.5 g/mol.
- So, the formula mass of KCl is
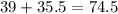 g/mol.
g/mol.
Therefore, 87.0 grams of KCl is equal to
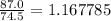 moles.
moles.
From the reaction, we know that for every 2 moles of KCl produced, 3 moles of diatomic oxygen are produced. This means that there are
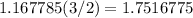 moles of diatomic oxygen.
moles of diatomic oxygen.
The formula mass of diatomic oxygen is 32 g/mol, meaning
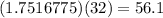 grams of diatomic oxygen were produced.
grams of diatomic oxygen were produced.