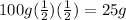Answer:
25g
Step-by-step explanation:
According to a quick internet search, the half-life of I-131 is 8 days.
The amount left after 16 days can be calculated with the radioactive exponential decay formula:
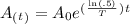
Where:
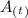 = the amount left as a function of time
= the amount left as a function of time
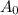 = the original amount (100g)
= the original amount (100g)
T = the half-life of the isotope (8d)
t = time (16d)
So:
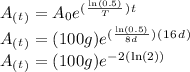
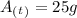
This answer is intuitive because the isotope has been through two half-lives: