Answer:
381.33 kg
Step-by-step explanation:
relative humidity is 54%, it means that when saturation vapor pressure is 100 pascals, partial vapor pressure is 54 pascals [taking Pascals as no unit is mentioned]
Now, using PV = nRT
⇒
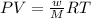
⇒
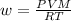
here input P = 54 Pascals, V = (18*9*6)X10^3 L, T = (25+273)°K
R = 8.314 Jmol^-1K^-1, M = mass of water in kg = 18X10^-3 kg