Answer:
Question 1. There are
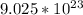 molecules in 270g of glucose.
molecules in 270g of glucose.
Question 2. There are
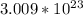 molecules in 40g of glucose.
molecules in 40g of glucose.
Step-by-step explanation:
Question 1. 270g of glucose, no. of molecules.
1. Find the chemical formula of glucose.
Formula:
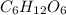
2. Find the molar mass of this compound.
Using the molar mass of each element, which can be found in the periodic table, we make the following calculations:
Molar mass of C: 12.011 u
Total mass of C in compound: (12.011 * 6)= 72.066
Molar mass of H: 1.008 u
Total mass of H in compound: (1.008 * 12)= 12.096
Molar mass of O: 15.999 u
Total mass of O in compound: (15.999 * 6)= 95.994
Why did we multiply the molar mass of C, H and O by 6, 12, and 6? This is because the formula contains 6, 12, and 6 atoms of each element, respectively.
Add up all masses:
72.066 + 12.096 + 95.994= 180.156 g/mole
This result means that 1 mole of glucose has a mass of 180.156 grams.
3. Calculate the number of molecules.
Furthermore, 1 mole of any compound has a total of
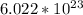 molecules. To find the amount of molecules, find the amount of moles and then the amount of molecules. Do it in the following fashion:
molecules. To find the amount of molecules, find the amount of moles and then the amount of molecules. Do it in the following fashion:
180.156g -----> 1 mole
270g -----------> x
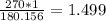
270 grams of glucose is the equivalent of 1.499 moles of said compound.
Now, find the number of molecules using the amount of moles calculated previously.
180.156g ---->
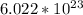
270g ---------> x
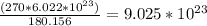
4. Conclusion.
There are
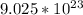 molecules in 270g of glucose.
molecules in 270g of glucose.
Question 2. 40g of ammonium nitrate, no. of molecules.
Repeat the same process explained previously:
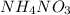
N: 14.007 u
N: 14.007 u
O: (15.999 * 3)= 47.997 u
14.007 + 4.032 + 14.007 + 47.997= 80.043 g/mole
80.043g ---->
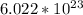
40g -----------> x
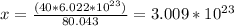
There are
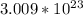 molecules in 40g of glucose.
molecules in 40g of glucose.