Answer:
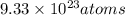
Explanations:
Given the following parameters:
Moles of Phosphorous = 0.620 moles
According to the compound, for every 2 moles of Phosphorous, there are 5 moles of Oxygen, hence the moles of oxygen contained is given as:
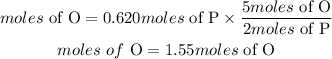
According to Avogadro's constant
1 mole = 6.02 * 10^23 atoms
1.55moles of O = 1.55 * 6.02 * 10^23 atoms
1.55moles of O = 9.33 * 10^23 atoms
Hence the individual oxygen atoms contained in a sample of P2O5 that also contains 0.620 moles of P is 9.33 * 10^23 atoms