ANSWER
The mass of the sample is 72.96 grams
Step-by-step explanation
Given that;
The heat lost is 6008 J
The temperature drop of the sample - 19.7 degrees Celcius
The specific heat capacity of water is 4.18J/g degrees Celcius
Follow the steps below to find the mass of the sample
Step 1; Write the formula for heat energy
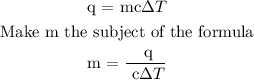
Step 2; Substitute the given data into the formula in step 1
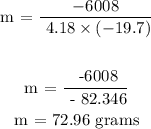
Therefore, the mass of the sample is 72.96 grams