1) Preparing a dilution
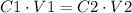
C1: Initial concentration.
V1: Initial volume.
C2: final concentration.
V2: final volume.
2) List known values
C1: 0.250 M sucrose
V1:
C2: 0.0310 M sucrose
V2: 400.0 mL
3) Plug in known values and solve for V2
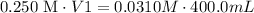
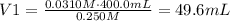
The solution should be made as follows
Add 49.6mL of 0.250M sucrose to 350.4mL of water to get 400.0mL of 0.0310 M sucrose.