The first statement can be written mathematically as
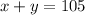
and the second statement can be written as
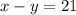
Hence, we have 2 equations in 2 unknows. We can solve this system by elimination:
If we add these equations, we have
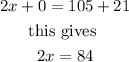
therfore,
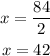
Since we know x, we can substitute this value into the first equation, It yields
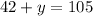
and now we can isolate y:
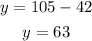
Finally, the answer is (63,42)