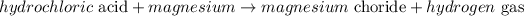Explanations:
When hydrochloric acid reacts with a metal, it will produce the chloride of the metal involved and hydrogen gas will evolve (single replacement reaction).
If the metal is magnessium, the reaction between hydrochloric acid and magnesium is expressed as:
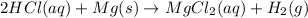
The word equation is given as: