1) Convert grams to moles
the molar mass of C2F4 is 100.0150 g/mol
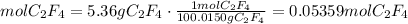
2) Convert moles of C2F4 to moles of F
F to C2F4 ratio
4 atoms of F: 1 molecule of C2F4
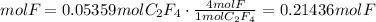
3) Convert moles of F to atom units of F
Avogadro's number is 6.022*10^23
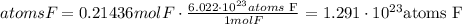
There are 1.291*10^23 atoms of Fluorine in 5.36 g of C2F4.