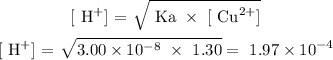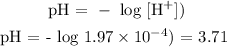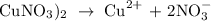
The NO3- ion is the conjugate base of a strong acid, it will not affecy the pH, but Cu2+ ion is not conjugate acid of a strong base, it will affect the pH.
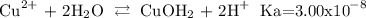
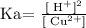
we can use the approximation that [Cu2+] = initial concentration of Cu(NO3)2 because the is Ka is very small
Solve for [H+]: