To determine the amplitude we need to see where is the middle line of the function, the middle line passes through the middle value of the y components of the maximum and minimum. Then the middle line passes through;
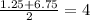
Now, the amplitude is the vertical distance between the maximum and the middle value, then in this case we have:
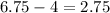
Therefore the amplitude is 2.75