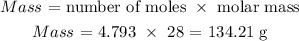Answer:
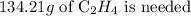
Step-by-step explanation:
Here, we want to calculate the mass of ethene needed
From the balanced equation of reaction:
1 mole of ethene needed 3 moles of oxygen
x moles of ethene will need 14.38 moles of oxygen
To get the value of x, we have it that:
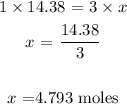
To get the mass needed, we have to multiply the number of moles by the molar mass of ethene
The molar mass of ethene is 28 g/mol
Thus, we have the needed mass as: