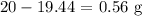Answer:
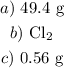
Step-by-step explanation:
a) Here, we want to get the mass produced
The mass produced will be dependent on the limiting reactant
The limiting reactant is the reactant that would produce the lesser amount of the product.
Firstly, we need to get the number of moles of the reactants
At any point in time, the number of moles could be calculated using:
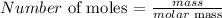
For Sodium, we have the atomic mass as 23 amu
Thus, we have the number of moles as:
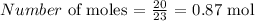
From the balanced equation of reaction, 2 moles of Na gave 2 moles of NaCl
This means 0.87 mol Na will give 0.87 mol NaCl
To get the mass of NaCl produced, we multiply the number of moles by the molar mass. The molar mass of NaCl is 58.5 g/mol
Thus, the mass would be:
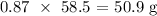
We repeat the same step for Chlorine molecule
The molar mass of the molecule is 71 g/mol
Thus, the number of moles that reacted will be:
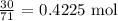
From the equation of reaction, 1 mole of chlorine gave 2 moles NaCl
The number of moles of NaCl produced from 0.4225 Cl2 will be:
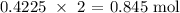
The mass that was produced from this would be:
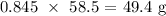
The lesser mass would be our answer which is 49.4 g
b) The limiting reactant produced the lesser amount of the product and we have that as Cl2
c) To get this, we have to convert the moles of Cl2 to the moles of Na
From the equation of reaction
1 mole of Cl2 reacted with 2 moles Na
0.4225 mole Cl2 will react with:
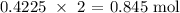
The mass that was produced from this will be:
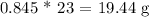
We started with 20 g and 19.44 g remained
The mass of excess reactant is thus: